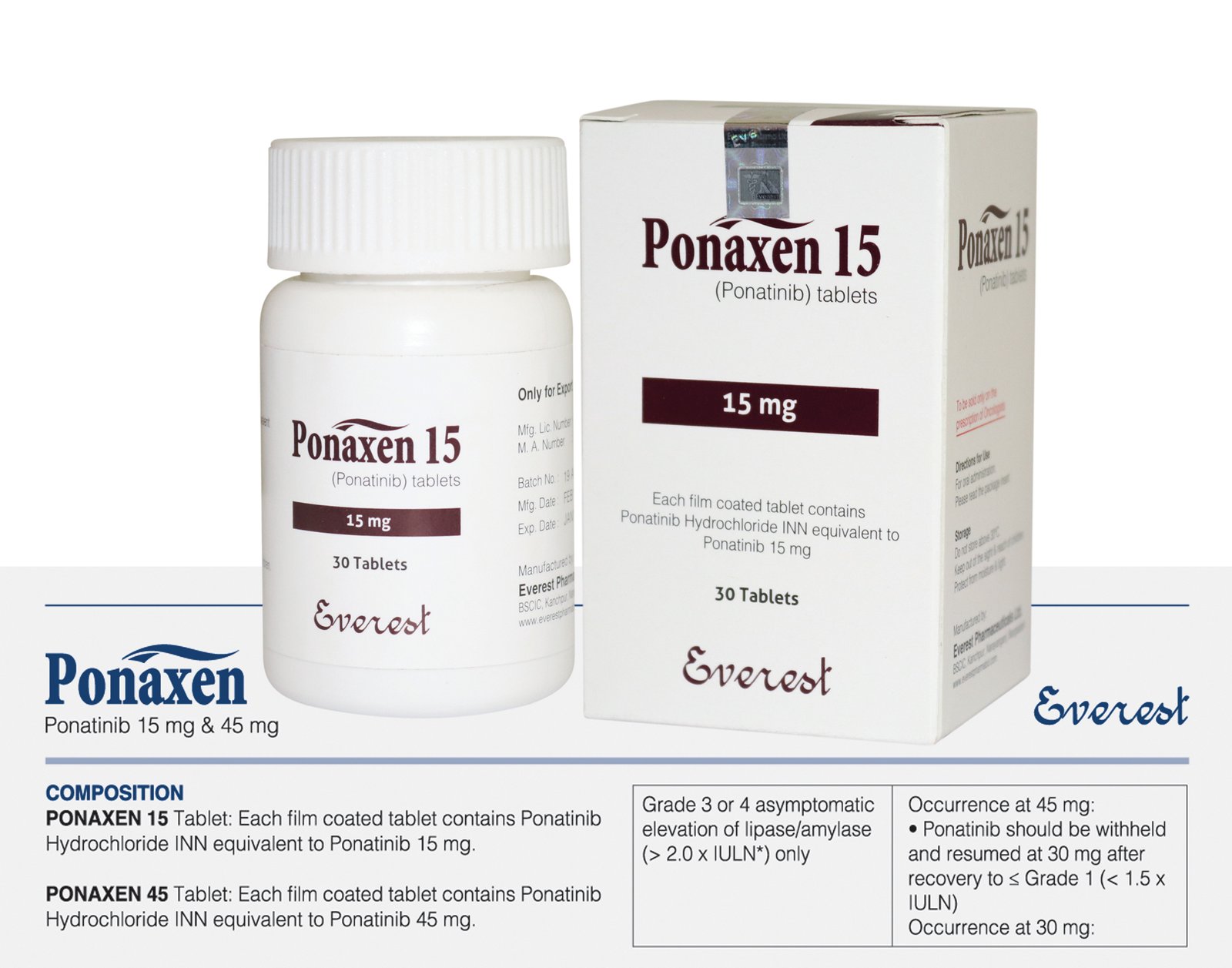
Ponatinib (Iclusig) 15 mg
Ponaxen, containing Ponatinib Hydrochloride, which is the International Nonproprietary Name (INN) equivalent to Ponatinib, is prescribed for chronic myeloid leukemia (CML) and Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) when prior treatments with tyrosine kinase inhibitors (TKIs) have failed or caused intolerance. As a TKI, Ponaxen targets BCR-ABL, a significant factor in CML and Ph+ ALL, hindering cell growth and prompting cell death, even in cases where resistance to other TKIs is present. The standard dosage is 15 mg orally once daily, with or without food, requiring careful monitoring due to potential drug interactions. Common adverse effects encompass arterial blood clotting, liver issues, and bone marrow suppression, while severe complications may involve heart failure and arterial blockages. In cases of overdose, supportive care is crucial, as specific antidotes are unavailable. Regular monitoring of liver and pancreatic enzymes is advised to address potential risks.
Ponatinib, marketed under the brand name Iclusig, is an oral prescription medication classified as a tyrosine kinase inhibitor (TKI). It is primarily used for the treatment of certain types of leukemia, specifically chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), especially in patients who are resistant or intolerant to other treatments. Ponatinib is particularly effective against leukemias with the T315I mutation—a mutation that confers resistance to many other TKIs.
Mechanism of Action
Tyrosine kinases are a class of enzymes that are essential for controlling cell division and proliferation, and ponatinib acts by targeting and blocking these enzymes. The aberrant BCR-ABL gene generates a tyrosine kinase that promotes unchecked cell division in malignancies such as CML and Ph+ ALL. By binding to and inhibiting this aberrant kinase, including its resistant variants like the T315I mutation, ponatinib stops the proliferation of cancer cells and causes their death.
Ponatinib has broad-spectrum anti-leukemic actions since it inhibits not only BCR-ABL but also VEGFR, FGFR, PDGFR, and Src family kinases.
Indications and Usage
The following circumstances allow for the usage of ponatinib (Iclusig):
Treatment of adult patients with chronic myeloid leukemia (CML) in the blast, rapid, or chronic phases who have not responded well to previous tyrosine kinase inhibitor therapy.
Treatment of adult patients with acute lymphoblastic leukemia (Ph+ ALL) that has been resistant to or intolerant to previous TKI therapy and has the Philadelphia chromosome.
Treatment of adult patients with T315I-positive Ph+ ALL or T315I-positive CML (chronic, rapid, or blast phase).
It is usually administered to patients who have not responded to prior treatments because of resistance or excessive side effects, rather than as a first-line treatment.
Dosage and Administration
There are several strengths of ponatinib tablets, including 15 mg, 30 mg, and 45 mg. The first dosage is frequently determined by the patient’s unique mutation profile and the stage of leukemia.
For the majority of individuals, the first dosage is 45 mg once daily.
For maintenance therapy or for individuals who see a notable response and are susceptible to adverse effects, a lesser dose, such as 15 mg once daily, may be utilized.
Frequently, dose modifications are necessary in response to side effects and treatment response.
Ponatinib can be taken with or without meals, and the tablets should be taken whole with water.
Precautions and Warnings
Ponatinib (Iclusig) 15 mg has a number of serious side effects, and patients need to be constantly watched during the course of treatment. Important safety measures consist of:
Cardiovascular Risks: Serious blood clots and blood channel narrowing brought on by ponatinib may result in peripheral artery disease, heart attacks, and strokes.
Hypertension: During treatment, elevated blood pressure should be managed because it is prevalent.
Pancreatitis: Pancreatic inflammation is a risk and may need stopping the medication.
Myelosuppression: Anemia, bleeding, and infection can all be made more likely by low blood counts (white, red, and platelets).
Pregnancy and Breastfeeding: Ponatinib should not be used during pregnancy due to the potential for fetal damage. It is not advised to breastfeed while undergoing treatment.
Patients should have complete blood counts, liver function testing, and a comprehensive cardiovascular evaluation prior to beginning Ponatinib.
Side Effects
Common side effects of Ponatinib include:
High blood pressure
Rash
Abdominal pain
Fatigue
Headache
Dry skin
Nausea
Constipation
Joint pain
Serious side effects requiring immediate medical attention include:
Signs of a heart attack or stroke include abrupt numbness, loss of breath, and chest pain.
Severe abdominal pain
Jaundice or dark urine (indicative of liver issues)
Vision problems
Unusual bleeding or bruising.
Drug Interactions
Ponatinib is metabolized by the liver enzyme CYP3A4. Drugs that inhibit or induce this enzyme can affect Ponatinib levels:
Inhibitors (e.g., ketoconazole, clarithromycin) can increase Ponatinib levels and risk of toxicity.
Inducers (e.g., rifampin, phenytoin) can decrease its effectiveness.
All medications, including over-the-counter pharmaceuticals and supplements, should be disclosed to the patient’s healthcare provider.
Storage and Handling
Ponatinib should be stored at room temperature and away from heat and moisture. Tablets should be kept in their original packaging until use, and any unused or expired medication should be disposed of properly, following local regulations.
Conclusion
For some forms of leukemia, ponatinib (Iclusig) 15 mg is a powerful and focused therapy option, particularly for patients who have few other options because they are resistant to or intolerant of earlier medications. Despite the substantial clinical advantages it provides, the possibility of major side effects must be carefully considered when using it. To guarantee safety and efficacy, routine monitoring, patient education, and dose management are crucial.
Order Now At Mdx Pharma bd….
To order from MDX Pharma BD, visit their website at https://mdxpharmabd.com, where you can browse products and place orders online. For inquiries or orders via email, contact emedicarepharma@gmail.com. Alternatively, call (+88) 01929123476. Their address is 29, Abdullahpur, Uttara, Dhaka-1230, Bangladesh.
1. What is the purpose of 15 mg of Ponatinib (Iclusig)?
Adults with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia (CML) are treated with ponatinib, particularly when they have a particular T315I mutation or are resistant to conventional tyrosine kinase inhibitors (TKIs).
2. Ponatinib: How does it work?
The BCR-ABL tyrosine kinase, including mutant versions like T315I that are frequently resistant to conventional treatments, is inhibited by ponatinib. This prevents cancer cells from proliferating and promotes apoptosis, or cell death.
3. How is 15 mg of Ponatinib taken?
As prescribed by a healthcare professional, it is taken orally once daily, with or without food.
4. What are Ponatinib’s typical side effects?
High blood pressure, rash, exhaustion, headache, constipation, abdominal pain, and dry skin are typical adverse effects.
5. Which major dangers are connected to ponatinib?
Blood clots, heart attacks, strokes, liver damage, pancreatitis, and extreme hypertension are among the serious dangers. Frequent observation is crucial.
6. Is it safe to use ponatinib when pregnant or nursing?
Ponatinib should not be used when pregnant or nursing since it may harm the fetus. It is advised to use effective contraception both during and after therapy.
| Product Name | Ponaxen |
|---|---|
| Generic Name | Ponatinib Hydrochloride INN equivalent to Ponatinib |
| Formulation | Tablet |
| Available Pack size | 30 Tablets |
| Strengths | 15 mg |

 Cart is empty
Cart is empty