Tivoxen (Tivozanib INN) 1.34 mg
0.00$
Tivoxen, featuring Tivozanib as its International Nonproprietary Name (INN), is indicated for the treatment of advanced renal cell carcinoma (RCC) in adult patients who have not responded to prior systemic therapy. Tivozanib, a tyrosine kinase inhibitor (TKI), disrupts angiogenesis by selectively targeting vascular endothelial growth factor receptors (VEGFR-1, -2, and -3), thereby impeding tumor progression. The recommended dose is 1.34 mg orally once daily, irrespective of food intake, with adjustments possible based on individual tolerance. Close monitoring for adverse effects such as fatigue, hypertension, and gastrointestinal disturbances is necessary, with prompt intervention and possible treatment interruption if severe reactions occur. Due to potential fetal harm, effective contraception is essential during and after treatment. Electrocardiographic monitoring is advised for patients with a history of cardiac issues or QT prolongation predisposition. In case of overdose, supportive care is recommended, with no specific antidote available. Therefore, prompt symptomatic treatment is vital.
- Regular blood pressure monitoring is recommended during Tivoxen treatment, with antihypertensive therapy initiated or adjusted as necessary.
- Patients should be monitored for signs of proteinuria, thyroid dysfunction, and hand-foot syndrome, with treatment interruption or discontinuation if these occur.
- Effective contraception is crucial during and after treatment due to the potential for fetal harm.
- Patients with cardiac history or predisposition to QT prolongation should undergo electrocardiographic monitoring during Tivoxen therapy.
Indication: Tivoxen, containing Tivozanib as the International Nonproprietary Name (INN), is indicated for treating advanced renal cell carcinoma (RCC) in adult patients who have previously undergone systemic therapy without success.
Pharmacology: Tivozanib, a tyrosine kinase inhibitor (TKI), selectively targets vascular endothelial growth factor receptors (VEGFR-1, -2, and -3), disrupting angiogenesis and inhibiting tumor progression.
Dosage and Administration: The recommended dose of Tivoxen is 1.34 mg orally once daily, regardless of food intake. Treatment should continue until disease progression or intolerable adverse effects emerge, with possible dose adjustments based on individual tolerance.
Interactions: Tivoxen may interact with drugs affecting cytochrome P450 enzymes, notably CYP3A4. Caution is advised when co-administering with potent CYP3A4 inhibitors or inducers. Additionally, vigilance is warranted when combining Tivoxen with medications prolonging the QT interval.
Side Effects: Common side effects include fatigue, hypertension, diarrhea, nausea, decreased appetite, and dysphonia. Other potential adverse reactions encompass proteinuria, hypothyroidism, and hand-foot syndrome, while serious events like hemorrhagic and arterial thromboembolic events are possible.
Precautions and Warnings:
Overdose Effect: In the event of overdose, provide supportive care and closely monitor for adverse reactions. No specific antidote exists; therefore, appropriate symptomatic treatment should be initiated as required.
| Product Name | Tivoxen |
|---|---|
| Generic Name | Tivozanib INN |
| Formulation | Capsule |
| Available Pack Size | 21 Capsules |
| Available Strength | 1.34 mg |

 Cart is empty
Cart is empty 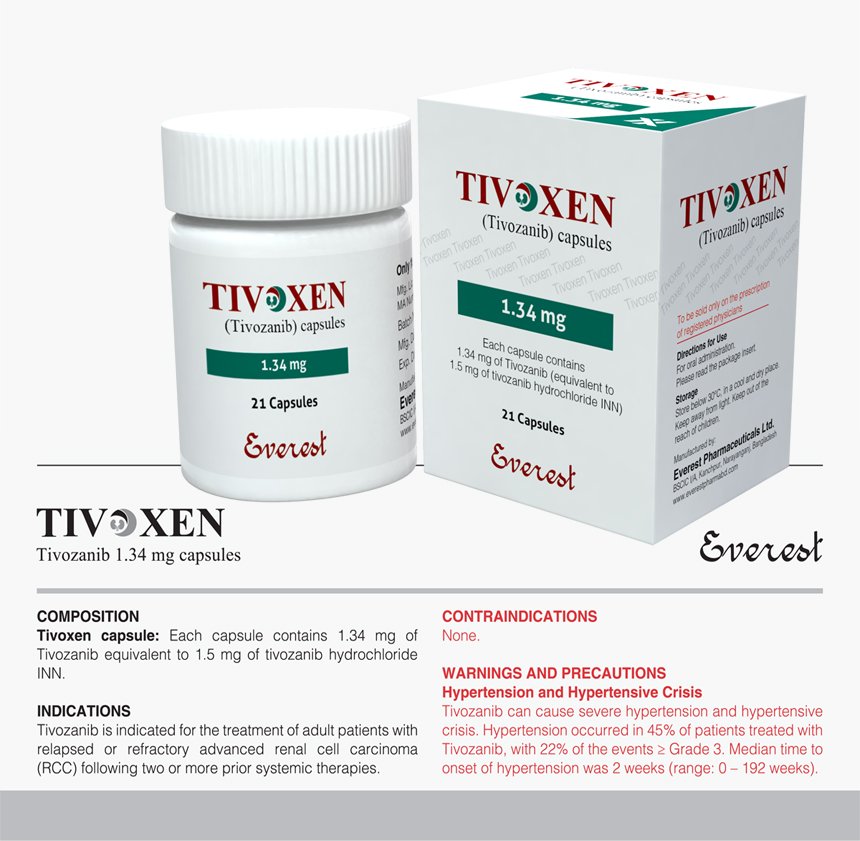



Reviews
There are no reviews yet.