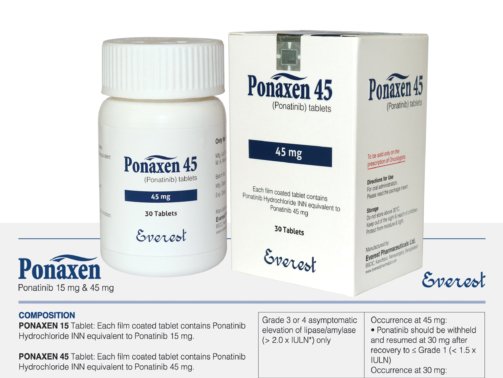
Sotorasib (Lumakras) 120 mg
0.00$
Sotorasib (INN) 120 mg is indicated for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) harboring KRAS G12C mutations. By irreversibly inhibiting this mutated form of the KRAS oncogene, sotorasib suppresses tumor growth. The recommended dosage is 960 mg orally once daily. While common side effects include diarrhea and nausea, serious adverse effects such as interstitial lung disease and hepatotoxicity may occur. Patients should be monitored for these risks, and in cases of overdose, supportive care should be administered.
Sotorasib is a targeted cancer medication used to treat non-small cell lung cancer (NSCLC) with a particular KRAS gene mutation known as KRAS G12C. Lumakras is the brand name under which it is offered. Lumakras, created by Amgen, is the first approved treatment to specifically target KRAS, a gene that was previously thought to be “undruggable,” marking a significant advancement in oncology. 120 mg of sotorasib is present in each tablet, which is normally taken orally once daily.
Mechanism of Action
In order to lock the KRAS G12C-mutated protein in an inactive GDP-bound form, sotorasib irreversibly inhibits it. This action prevents the KRAS protein from sending signals that promote cancer cell growth and survival. By blocking the mutant KRAS protein, Lumakras helps slow or stop the proliferation of cancer cells. This targeted approach leads to fewer side effects compared to traditional chemotherapy, which attacks both healthy and cancerous cells.
Indications and Usage
Adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a KRAS G12C mutation who have had at least one previous systemic therapy are eligible to receive sotorasib. About 25% of NSCLC cases include KRAS mutations, with the G12C variation making up about 13% of these cases. Once the KRAS G12C mutation has been confirmed by genetic testing, Lumakras is administered.
Dosage and Administration
The recommended dose of Lumakras is 960 mg once daily, which equals eight 120 mg tablets taken orally. Instead of chewing, crushing, or splitting tablets, they should be consumed whole. Lumakras can be taken with or without food, but they should be taken at the same time every day to maintain consistent levels of the drug in the body.
If a dose is missed by more than 6 hours, patients should skip the dose and take the next one at the regular time. Double dosing should be avoided.
Packaging and Presentation
Bottles of Sotorasib (Lumakras) 120 mg contain 240 tablets, which is enough for a 30-day supply when taking the medication as prescribed. To ensure recognition, the film-coated, oval-shaped, yellow tablets have the stamp “AMG 510.”
Side Effects and Warnings
Lumakras may have adverse effects, just like any other medicine. The most common adverse reactions include:
Diarrhea
Nausea
Fatigue
Hepatotoxicity (liver enzyme elevation)
Musculoskeletal pain
Cough
In clinical trials, hepatotoxicity was one of the most concerning side effects and required regular monitoring of liver function tests. Dose adjustments or interruptions may be necessary in cases of elevated liver enzymes or other severe adverse reactions.
Serious warnings may include:
Hepatotoxicity: Regular monitoring of liver function is crucial during treatment.
Interstitial lung disease (ILD)/pneumonitis: Although rare, this potentially fatal side effect requires immediate discontinuation of treatment if suspected.
Patients should inform their healthcare provider about any existing medical conditions, especially liver disease, and all medications they are taking to avoid harmful drug interactions.
Contraindications and Drug Interactions
The use of Lumakras with medications that are potent CYP3A4 inducers, like rifampin or St. John’s wort, should be done with caution since they may lessen the efficiency of sotorasib. However, there are no absolute contraindications listed. Similarly, sotorasib absorption may be hampered by acid-reducing substances such as proton pump inhibitors and antacids, requiring careful timing or other treatment strategies.
Clinical Effectiveness
Clinical results from Lumakras in the CodeBreaK 100 experiment were encouraging. The median response duration was 10 months, and the overall response rate (ORR) was roughly 36%. Patients with previously treated KRAS G12C-mutated NSCLC who had few options before Lumakras became available saw a considerable improvement in their outcomes.
Storage and Handling
To prevent moisture damage, lumakras should be kept in their original container with the desiccant at room temperature (20°C to 25°C / 68°F to 77°F). When not in use, bottles should be kept tightly closed and out of children’s reach.
Availability and Status of Prescriptions
120 mg of sotorasib (Lumakras) requires a prescription and can only be given by authorized medical professionals. Before starting treatment, genetic testing for the KRAS G12C mutation is required to guarantee proper use.
Conclusion
A significant development in precision oncology, sotorasib (Lumakras) 120 mg provides patients with non-small cell lung cancer with a KRAS G12C mutation with a targeted therapeutic option. Lumakras is establishing a new benchmark in individualized cancer treatment thanks to its easy oral dosage schedule, favorable side effect profile when compared to conventional medicines, and proven effectiveness in clinical trials. Close collaboration between patients and healthcare professionals is necessary to track treatment, control adverse effects, and maximize results.
Order Now At Mdx Pharma bd….
To order from MDX Pharma BD, visit their website at https://mdxpharmabd.com, where you can browse products and place orders online. For inquiries or orders via email, contact emedicarepharma@gmail.com. Alternatively, call (+88) 01929123476. Their address is 29, Abdullahpur, Uttara, Dhaka-1230, Bangladesh.
1. What is the purpose of sotorasib (Lumakras)?
Adults with non-small cell lung cancer (NSCLC) whose tumors have a KRAS G12C mutation and who have had at least one previous systemic therapy are treated with sotorasib.
2. How is sotorasib administered?
In response, sotorasib inhibits KRAS G12C. It functions by specifically focusing on and permanently blocking the mutant KRAS G12C protein, which is in charge of promoting the proliferation of cancer cells in some forms of non-small cell lung cancer.
3. What is the typical sotorasib dosage?
The suggested dosage is eight 120 mg tablets, or 960 mg taken orally once daily. With or without food, the tablets should be taken daily at the same time.
4. Does taking sotorasib include any significant risks?
In response, “Yes.” Among the grave dangers are:
Hepatotoxicity, or harm to the liver
Pneumonitis or interstitial lung disease (ILD)
severe adverse effects on the gastrointestinal tract
Lung symptoms and liver function should be routinely checked on patients.
5. What happens if I forget to take my Sotorasib dose?
Take the missed dose as soon as you remember if it has been less than six hours. Skip the missed dose and take the following one at your regular schedule if more than six hours have gone by. Avoid taking two doses at once.
6. Is it safe to take sotorasib when pregnant or nursing?
A fetus may be harmed by sotorasib. During treatment and for one week following the last dose, women who are capable of becoming pregnant should use an effective form of birth control. It is not advised to breastfeed while receiving therapy.
| Product Name | Sotoxen |
|---|---|
| Generic Name | Sotorasib INN |
| Formulation | Tablet |
| Available Pack size | 56 Tablets |
| Strengths | 120 mg |

 Cart is empty
Cart is empty