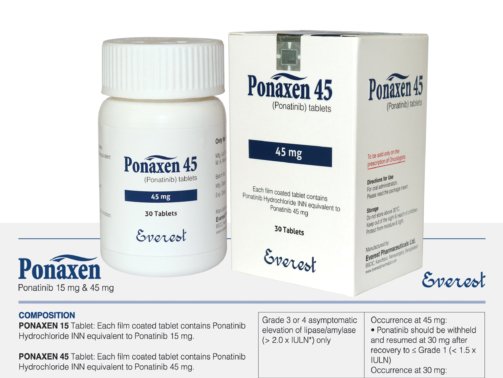
Ponatinib 15 mg (Iclusig)
0.00$
Ponatinix, an oral medication containing the generic drug Ponatinib, is approved for the initial treatment of individuals diagnosed with chronic myelogenous leukemia (CML).
It is a potent and multi-targeted third-generation Bcr-Abl tyrosine kinase inhibitor (TKI) recommended for adult patients with chronic phase, accelerated phase, or blast phase CML or Ph+ALL who have not received prior TKI therapy.
Additionally, it is prescribed for adult patients with T315I-positive CML (in chronic phase, accelerated phase, or blast phase) or T315I-positive Philadelphia chromosome-positive acute lymphoblastic leukemia
Ponatinib, marketed under the brand name Iclusig, is a potent oral tyrosine kinase inhibitor (TKI) used in the treatment of specific types of leukemia. It is especially effective in targeting the BCR-ABL fusion gene, including mutations that render other TKIs ineffective. The 15 mg formulation of Ponatinib is typically used in carefully adjusted dosing regimens tailored to the patient’s response and tolerance.
Indications
The main conditions for which ponatinib is prescribed include:
Chronic Myeloid Leukemia (CML): Particularly in individuals who have not responded well to previous TKI treatment.
Positive for the Philadelphia Chromosome Patients who have not responded to previous TKIs may develop acute lymphoblastic leukemia (Ph+ ALL).
Patients are especially well-suited for Ponatinib therapy if they have the T315I mutation, which results in resistance to the majority of other TKIs.
Mechanism of Action
BCR-ABL tyrosine kinase, a protein generated by the Philadelphia chromosomal abnormalities seen in CML and Ph+ ALL, is inhibited by ponatinib. Ponatinib is special and frequently used when other treatments don’t work because, unlike other TKIs, it works even against the T315I mutation.
Apart from BCR-ABL, ponatinib inhibits a number of kinases, such as VEGFR, FGFR, PDGFR, and SRC family kinases, that are essential for the growth and survival of cancer cells. Both its effectiveness and adverse effect profile are influenced by this multi-targeted activity.
Dosage and Administration
Ponatinib’s usual starting dose is 45 mg once day, however based on the patient’s reaction, any adverse effects, or particular treatment objectives, the dosage may be lowered to 30 mg or 15 mg. The 15 mg dosage is frequently used as a maintenance dose or in patients who require a lower dosage due to underlying risk factors or increased medication sensitivity.
Ponatinib tablets should be taken once day, with or without food. To guarantee adequate absorption, patients should swallow the pills whole, without chewing or crushing them.
Pharmacokinetics
Once-daily dose is supported by ponatinib’s high oral absorption and around 24-hour half-life. Since CYP3A4 is primarily responsible for its metabolism, co-administration with potent CYP3A4 inducers or inhibitors should be handled with caution. The medication has little renal clearance and is mostly eliminated through stool.
Effectiveness
Clinical studies have shown that Ponatinib works well for patients with Ph+ ALL and extensively pretreated CML, including those with the T315I mutation. Ponatinib demonstrated significant cytogenetic and molecular responses in the PACE trial, especially in patients who were intolerant or resistant to previous TKIs.
Safety and Side Effects
While Ponatinib is a powerful therapy, it is associated with several potential side effects, some of which can be serious. Common side effects include:
Hypertension
Rash
Abdominal pain
Fatigue
Headache
Constipation
Dry skin
More serious adverse events include:
Arterial occlusive events (e.g., heart attacks, strokes)
Venous thromboembolism
Hepatotoxicity
Pancreatitis
Heart failure
Myelosuppression
Because of these risks, careful monitoring is essential, and patients must be evaluated regularly for blood pressure, liver function, pancreatic enzymes, and signs of vascular complications. A boxed warning for hepatotoxicity, cardiac failure, and vascular occlusion is included by the FDA.
Precautions and Contraindications
Ponatinib 15 mg (Iclusig) is not recommended for use in:
Patients with a history of serious arterial or venous thrombosis unless the benefits clearly outweigh the risks.
Patients with uncontrolled hypertension.
Women who are pregnant or breastfeeding, as it can cause harm to the fetus or nursing infant.
Effective contraception is advised for women of reproductive potential during treatment and for some time after the last dose.
Drug Interactions
As Ponatinib is metabolized by CYP3A4, caution should be used with medications that are strong CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin) or inducers (e.g., rifampin, phenytoin), as they may alter drug levels. Grapefruit juice should also be avoided for the same reason.
Conclusion
Ponatinib 15 mg (Iclusig) is a targeted cancer treatment that is specifically used to treat Ph+ ALL and resistant or advanced CML. It differs from other TKIs in that it can block the T315I mutation. Its use must be carefully weighed against the possibility of severe negative effects, though. To optimize its benefits and reduce dangers, careful patient selection, dose modifications, and continuous monitoring are essential. For patients with few options because of resistance or intolerance to conventional medicines, Ponatinib provides hope as part of a customized therapy regimen.
Order Now At Mdx Pharma bd….
To order from MDX Pharma BD, visit their website at https://mdxpharmabd.com, where you can browse products and place orders online. For inquiries or orders via email, contact emedicarepharma@gmail.com. Alternatively, call (+88) 01929123476. Their address is 29, Abdullahpur, Uttara, Dhaka-1230, Bangladesh.
1. What is the purpose of Ponatinib (Iclusig)?
Adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia (CML) who are intolerant of or resistant to previous treatments are treated with ponatinib.
2. Ponatinib: How does it work?
The BCR-ABL protein, which stimulates the proliferation of cancer cells in CML and Ph+ ALL, is blocked by the tyrosine kinase inhibitor ponatinib.
3. What is the usual Ponatinib dosage?
45 mg once daily is the usual starting dose. Lower dosages, such 15 mg, could be utilized for people who are experiencing side effects or are in remission.
4. What are Ponatinib’s typical side effects?
High blood pressure, rash, exhaustion, headache, abdominal pain, and dry skin are typical adverse effects. Heart issues, liver damage, and blood clots are serious hazards.
5. Can cardiovascular problems be brought on by ponatinib?
A black box warning for major cardiovascular events, such as heart attacks, strokes, and blood clots, is associated with ponatinib. Frequent observation is crucial.
6. Is it safe to use ponatinib when pregnant?
An unborn child may be harmed by ponatinib. Both during and for a while after treatment, effective contraception is necessary.
| Product Name : | Ponatinix |
|---|---|
| Generic Name : | Ponatinib |
| Formulation : | Tablet |
| Available Pack Size : | 60’s pot |
| Available Strength : | 15 mg, 45 mg |

 Cart is empty
Cart is empty