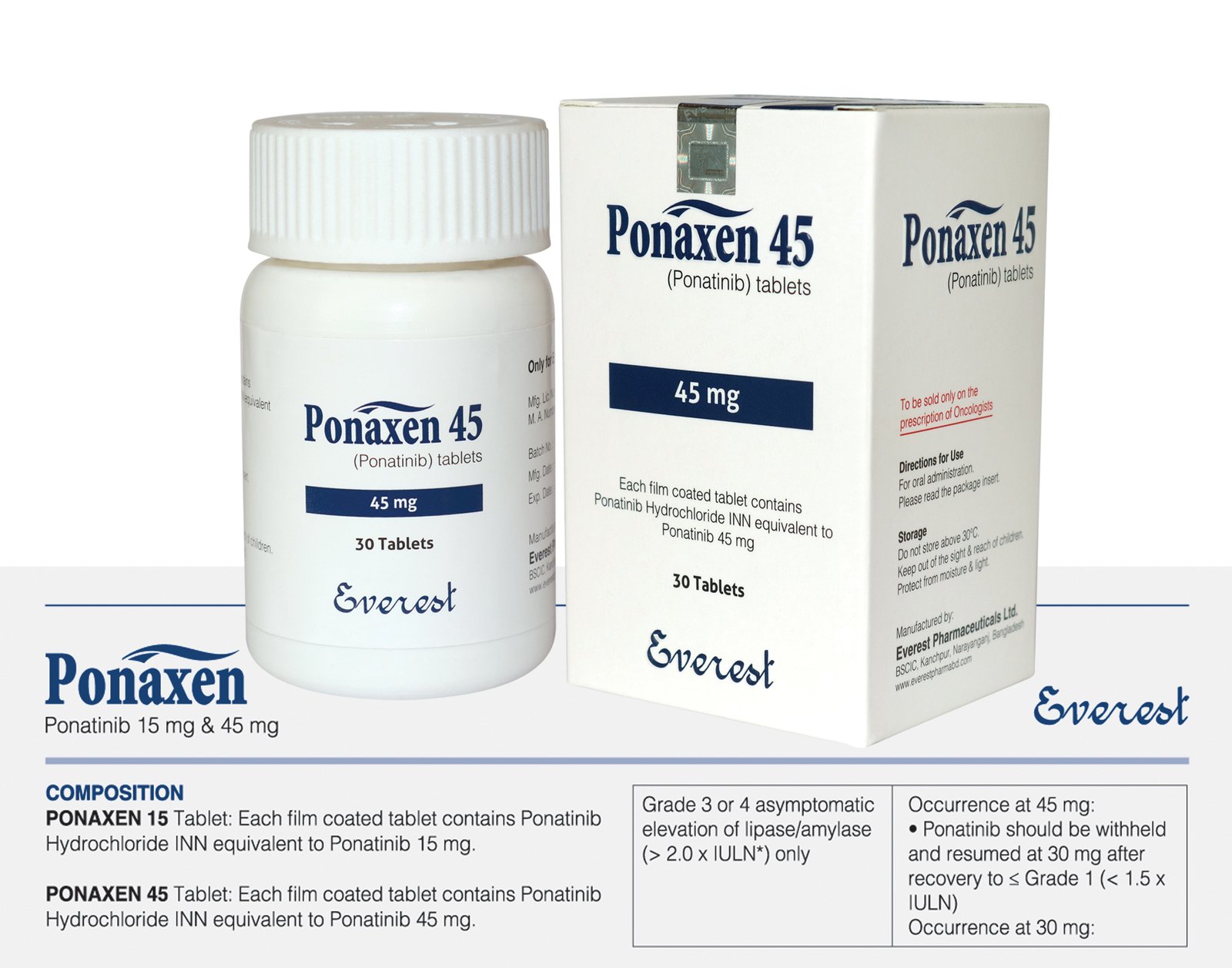
A prescription drug called ponatinib, which is sold under the brand name Iclusig, is used to treat acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML), two forms of blood cancer. It is a member of the tyrosine kinase inhibitor (TKI) medicine class and works especially well for patients with the T315I genetic mutation, which makes many other TKIs useless.
Ponatinib is usually taken orally once day at a dosage of 45 mg, which is the usual starting dose for many adult patients.
Mechanism of Action:
Ponatinib functions by inhibiting the activity of the Philadelphia chromosome-produced tyrosine kinase enzyme, the BCR-ABL protein, which is a genetic defect present in the majority of CML and certain ALL patients. The unchecked growth of white blood cells is caused by this protein. BCR-ABL and several other mutant forms, including as the T315I mutation, which is resistant to the majority of other TKIs like imatinib, dasatinib, and nilotinib, are inhibited by ponatinib.
Ponatinib inhibits the growth of malignant cells and restores normal cell growth by focusing on this abnormal signaling pathway.
Indications:
Adult patients with the following conditions can use 45 mg of ponatinib:
CML, or chronic myeloid leukemia:
Phase chronic (CP-CML)
Phase of acceleration (AP-CML)
Phase of blast (BP-CML)
especially when there has been intolerance or resistance to earlier TKI treatment.
Acute lymphoblastic leukemia with the Philadelphia chromosome (Ph+ ALL):
for individuals who have not responded well to prior TKI therapies.
Individuals that have the mutation T315I:
irrespective of the illness’s stage or previous medical procedures.
Administration & Dosage:
Ponatinib (Iclusig) 45 mg is usually started at a dose of 45 mg once daily. With or without food, the tablet must be consumed whole.
Depending on this, dosage changes can be required.
Side effects
Results from the laboratory
Co-occurring illnesses
In order to control adverse effects and preserve therapeutic efficacy, medical professionals may occasionally lower the dosage to 30 mg or 15 mg per day.
Crucial Safety Details:
Black Box Warning: Because of the possibility of hepatotoxicity, heart failure, venous thrombosis, and arterial occlusive events, ponatinib carries a boxed warning. Throughout their treatment, patients need to be constantly observed.
Common Side Effects:
Elevated blood pressure, or hypertension
Rash
Pain in the abdomen
A headache
Weariness
Skin that is dry
Vomiting and feeling queasy
Diarrhea or constipation
Joint or muscle pain
Serious Adverse Reactions:
Strokes and thrombosis
A heart attack
Toxicology of the liver
Pancreatitis
Bleeding (severe bleeding)
Myelosuppression, or decreased neutrophil numbers
Requirements for Monitoring: Patients taking ponatinib should have routine
monitoring of blood pressure
Total blood counts (CBC)
Tests of liver function
Tests for pancreatic enzymes, such as lipase
Cardiovascular evaluations
Contraindications and Precautions:
Because of the possible risks to the fetus or baby, it is not advised during pregnancy or when nursing.
Patients should exercise caution if they have a history of cardiovascular illness, high blood pressure, or pancreatitis.
Grapefruit and grapefruit juice should be avoided by patients while undergoing treatment since they may raise blood levels of Ponatinib and increase the risk of toxicity.
Drug Reactions:
The CYP3A4 enzyme metabolizes ponatinib. The safety and efficacy of ponatinib may be changed by medications that interfere with this enzyme.
CYP3A4 Inhibitors (raise levels of Ponatinib):
Ketoconazole
Clarithromycin
Ritonavir
CYP3A4 inducers, which reduce ponatinib levels:
Rifampin
Carbamazepine
Phenytoin
All drugs, vitamins, and herbs should be disclosed to the patient’s healthcare provider.
Storage and Handling:
Keep between 20°C and 25°C (68°F and 77°F) at room temperature.
The tablets should be kept in their original container with the cap securely fastened.
Keep out of children’s reach.
Conclusion:
A potent targeted treatment for resistant types of leukemia, particularly those involving the T315I mutation, is ponatinib (Iclusig) 45 mg. Despite being quite effective, it has a lot of hazards and needs to be closely watched by medical professionals. The advantages and possible drawbacks of Ponatinib should be thoroughly discussed with the patient’s physician before beginning treatment.
Order Now At Mdx Pharma bd….
To order from MDX Pharma BD, visit their website at https://mdxpharmabd.com, where you can browse products and place orders online. For inquiries or orders via email, contact emedicarepharma@gmail.com. Alternatively, call (+88) 01929123476. Their address is 29, Abdullahpur, Uttara, Dhaka-1230, Bangladesh.
1. What is the purpose of Ponatinib (Iclusig)?
A tyrosine kinase inhibitor called ponatinib is used to treat leukemias such Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) and chronic myeloid leukemia (CML), especially in patients who are intolerant or resistant to conventional treatments.
2. Ponatinib: How does it work?
The tyrosine kinases BCR-ABL and others are inhibited by ponatinib. It works particularly well against cells that have the T315I mutation, which makes them resistant to a lot of other TKIs.
3. What is Ponatinib’s suggested beginning dosage?
Although the amount may be changed depending on response and tolerability, the usual starting dose is 45 mg once daily.
4. How should I take 45 mg of ponatinib?
One oral dose each day, with or without food, is the recommended course of action. Instead of breaking or shattering the tablet, swallow it whole.
5. Is it appropriate to use Ponatinib when pregnant or nursing?
Ponatinib may be harmful to the fetus. During treatment and for six days following the final dose, women should refrain from nursing and utilize effective contraception.
| Product Name | Ponaxen |
|---|---|
| Generic Name | Ponatinib Hydrochloride INN equivalent to Ponatinib |
| Formulation | Tablet |
| Available Pack size | 30 Tablets |
| Strengths | 45 mg |

 Cart is empty
Cart is empty