Panovir(Sofosbuvir+Velpatasvir ) 100 mg
0.00$
It is a fixed-dose combination tablet containing sofosbuvir and velpatasvir for oral administration. Sofosbuvir is a nucleotide analog HCV NS5B polymerase inhibitor and velpatasvir is an NS5A inhibitor.
Indications
Sofosbuvir and Velpatasvir combination is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) genotype 1, 2, 3, 4, 5 or 6 infection
– without cirrhosis or with compensated cirrhosis
– with decompensated cirrhosis for use in combination with ribavirin
Dosage & Administration
The recommended dosage is one tablet (400 mg of Sofosbuvir and 100 mg of Velpatasvir) taken orally once daily. Recommended treatment regimen:
– Patients without cirrhosis and patients with compensated cirrhosis (Child-Pugh A) – one tablet once daily for 12 weeks
– Patients with decompensated cirrhosis (Child-Pugh B or C) – one tablet once daily and Ribavirin for 12 weeks. The recommended dosage of Ribavirin is based on bodyweight (1000 mg/day for patients < 75 kg and 1200 mg/day for ≥ 75 kg, in two divided dose/day)
No dosage recommendation can be given for patients with severe renal impairment (eGFR ≤ 30 mL/min/1.73 m2) or with ESRD, due to higher exposures of the predominant sofosbuvir metabolite.
Side Effects
The most common side effects of Sofosbuvir and Velpatasvir combination include headache and tiredness. Treatment may result in slowing of the heart rate along with other symptoms when taken with amiodarone (a medicine used to treat certain heart problems).
Precautions
Serious symptomatic bradycardia may occur in patients taking amiodarone, particularly in patients also receiving beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease. Coadministration of amiodarone with Sofosbuvir and Velpatasvir combination is not recommended. In patients without alternative viable treatment options, cardiac monitoring is recommended.
Use in Pregnancy & Lactation
No adequate human data are available to establish whether or not Sofosbuvir and Velpatasvir combination poses a risk to pregnancy outcomes. If Sofosbuvir and Velpatasvir combination administered with Ribavirin, the combination regimen is contraindicated in pregnant women and in men whose female partner is pregnant or going to be pregnant in next six months.
Drug Interaction
Drugs may decrease the concentrations of sofosbuvir and/or velpatasvir: Antacids, H2-receptor antagonists, Proton-pump inhibitors etc.
Coadministration is not recommended with: topotecan, Carbamazepine, Phenytoin, Phenobarbital, Oxcarbazepine, Rifabutin, Rifampin, Rifapentine, efavirenz, Tipranavir, Ritonavir, Hypericum perforatum.
Coadministration of Sofosbuvir and Velpatasvir combination, with Rosuvastatin, Atorvastatin may significantly increase the concentration of Rosuvastatin, Atorvastatin.
Over Dose
If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose includes monitoring of vital signs as well as observation of the clinical status of the patient.
Storage
Store in a cool and dry place (preferably below 30°C). Keep out of reach of children.
Commercial Pack
Panovir Blister Pack: Each box contains 1 blister strip of 6 tablets.
Panovir Container Pack: Each bottles contains 28 tablets.
Find A Product
Other products from Anti Viral, Hepatitis C Medication
Panovir
Sofosbuvir+Velpatasvir
Virodacla
Daclatasvir

 Cart is empty
Cart is empty 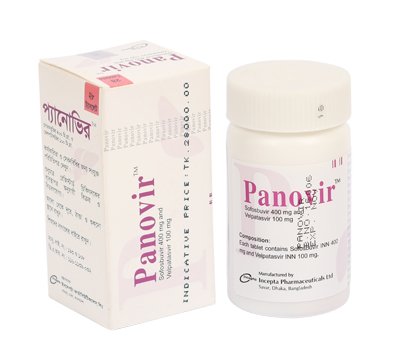

Reviews
There are no reviews yet.