Daconib (Dacomitinib INN) 45 mg
0.00$
Daconib, also known as Dacomitinib, is indicated as the first-line therapy for metastatic non-small cell lung cancer (NSCLC) patients with specific EGFR mutations. Acting as a potent inhibitor of human EGFR tyrosine kinase, Daconib obstructs tumor cell proliferation and metastasis. The recommended dosage is 45 mg orally once daily, and caution is advised regarding interactions with drugs affecting cytochrome P450 enzymes. Side effects may include diarrhea, rash, and potential severe reactions such as interstitial lung disease or hepatotoxicity. Regular monitoring for adverse effects is essential, with supportive care recommended in case of overdose due to the absence of a specific antidote.
- Patients should undergo monitoring for signs of interstitial lung disease, with treatment interruption or discontinuation if suspected.
- Regular liver function tests are advised during Daconib treatment to monitor for hepatotoxicity.
- Ophthalmic monitoring is recommended due to potential ocular disorders.
- Patients should be informed about the risk of cutaneous reactions, with treatment cessation if severe reactions occur.
Indication: Daconib, also recognized as Dacomitinib under the International Nonproprietary Name (INN), is indicated as the initial therapy for patients diagnosed with metastatic non-small cell lung cancer (NSCLC) carrying either epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R substitution mutations.
Pharmacology: Daconib functions as a potent and irreversible inhibitor of human EGFR tyrosine kinase. By binding to EGFR’s ATP-binding site, it blocks autophosphorylation and downstream signaling pathways, effectively hindering tumor cell proliferation, angiogenesis, and metastasis.
Dosage and Administration: The recommended dosage of Daconib is 45 mg orally once daily, administered continuously until disease progression or unacceptable adverse reactions occur. It can be taken with or without food, with dosage adjustments as per individual patient tolerance.
Interactions: Daconib may interact with medications affecting cytochrome P450 enzymes, particularly CYP3A4. Caution is advised when co-administering with potent CYP3A4 inhibitors or inducers. Additionally, care should be taken when combining Daconib with drugs prolonging the QT interval.
Side Effects: Typical side effects of Daconib include diarrhea, rash, acneiform dermatitis, paronychia, stomatitis, and decreased appetite. Severe adverse reactions might involve interstitial lung disease, hepatotoxicity, ocular issues, and cutaneous reactions like Stevens-Johnson syndrome.
Precautions and Warnings:
Overdose Effect: In instances of overdose, supportive care is essential. No specific antidote exists for Daconib overdose, hence close monitoring for adverse reactions is crucial, with appropriate symptomatic treatment as needed.
| Product Name | Daconib |
|---|---|
| Generic Name | Dacomitinib INN |
| Formulation | Tablet |
| Available Pack Size | 30 Tablets |
| Available Strength | 45 mg |

 Cart is empty
Cart is empty 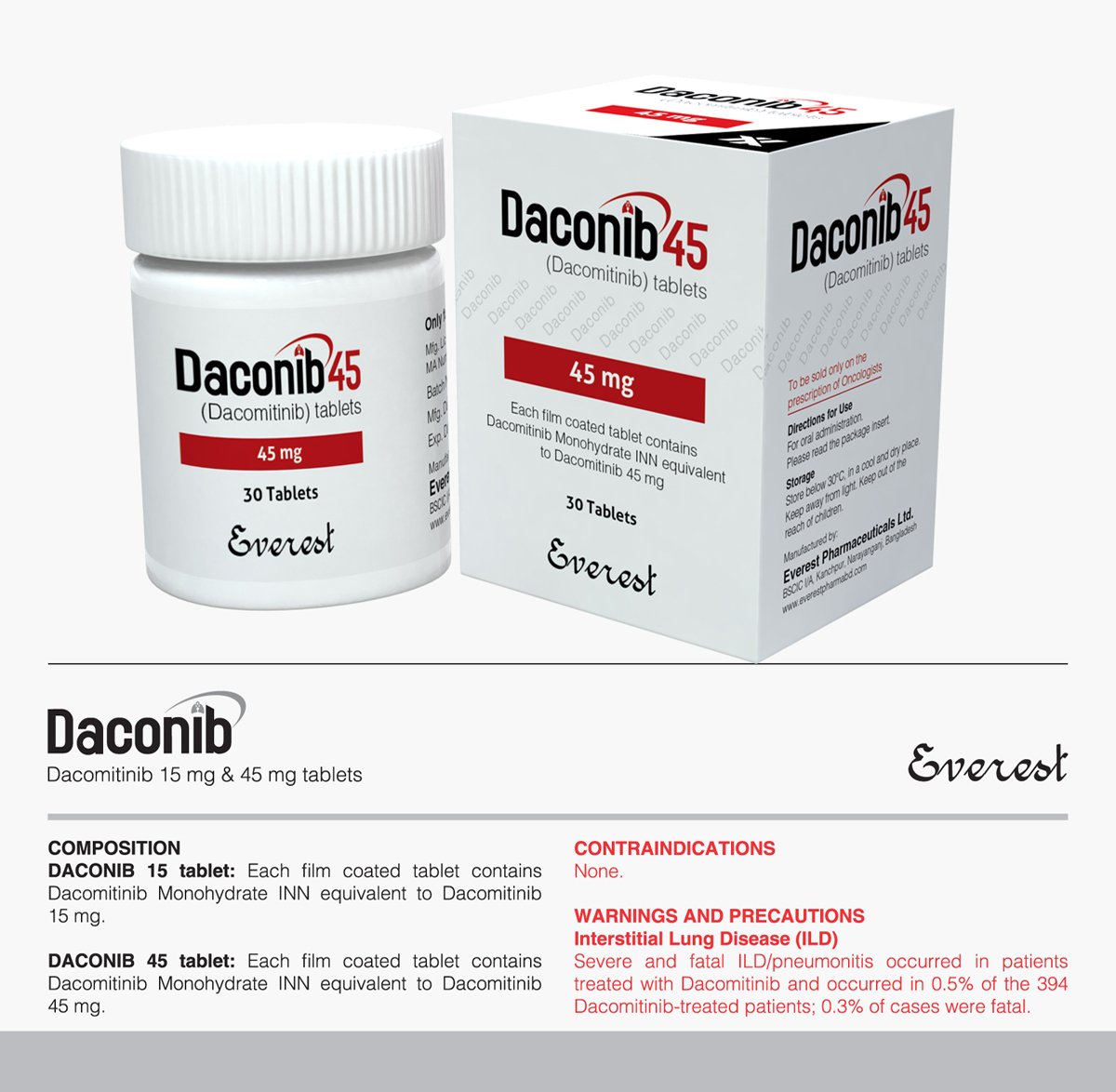


Reviews
There are no reviews yet.