Ascimib (Asciminib) 40 mg
0.00$
Ascimib, also known as Asciminib, is indicated for adults with chronic myeloid leukemia (CML) resistant or intolerant to previous tyrosine kinase inhibitor therapies. It selectively inhibits the BCR-ABL1 kinase, disrupting downstream signaling pathways vital for leukemic cell proliferation. Administered orally at a dosage of 40 mg twice daily, adjustments may be made based on individual responses. Caution is advised regarding interactions with CYP3A4-metabolized drugs. Side effects include fatigue, gastrointestinal disturbances, myelosuppression, and elevated liver enzymes. Regular monitoring for adverse effects like QT prolongation and pancreatitis is essential. In case of overdose, supportive care is recommended. Patients should seek personalized medical advice regarding Ascimib usage.
- Patients should be regularly monitored for signs of myelosuppression, necessitating adjustments or interruptions in treatment based on hematologic parameters.
- Due to its potential to prolong the QT interval, electrocardiogram monitoring is recommended, especially for patients with a cardiac history.
- Ascimib use has been associated with pancreatitis; hence, patients should be closely observed for related symptoms, with treatment cessation if suspected.
- As Ascimib may induce embryo-fetal toxicity, effective contraception is advised during treatment and for at least 30 days after the last dose, particularly in females of reproductive potential.
Indication: Ascimib, also referred to as Asciminib, in a 40 mg dosage, is approved for managing chronic myeloid leukemia (CML) in adults who have developed resistance or intolerance to previous tyrosine kinase inhibitor (TKI) therapies.
Pharmacology: Ascimib acts as a selective inhibitor of the BCR-ABL1 kinase, pivotal in CML’s pathogenesis. By binding selectively to the ABL myristoyl pocket, Ascimib inhibits the BCR-ABL1 oncoprotein’s activity, disrupting downstream signaling pathways crucial for leukemic cell proliferation.
Dosage and Administration: The recommended dose of Ascimib is 40 mg orally twice daily, preferably taken at least two hours before or after meals. Adjustments in dosage might be necessary based on individual patient responses and tolerances.
Interactions: Ascimib could interact with medications metabolized by CYP3A4 and those affecting P-glycoprotein. Caution is warranted when co-administering with potent CYP3A4 inhibitors or inducers. Vigilant monitoring is advised when combining Ascimib with other drugs.
Side Effects: Common side effects of Ascimib encompass fatigue, gastrointestinal disturbances like nausea, diarrhea, and abdominal discomfort, myelosuppression leading to thrombocytopenia, neutropenia, and anemia, along with elevated liver enzymes. Serious adverse reactions may involve hemorrhage, QT prolongation, and pancreatitis.
Precautions and Warnings:
Overdose Effect: In instances of Ascimib overdose, supportive care is advocated. No specific antidote exists; thus, close monitoring for adverse reactions is essential, with appropriate symptomatic treatment initiated as needed.
| Product Name | Ascimib |
|---|---|
| Generic Name | Asciminib |
| Formulation | Tablet |
| Available Pack Size | 30 Tablets |
| Available Strength | 40 mg |

 Cart is empty
Cart is empty 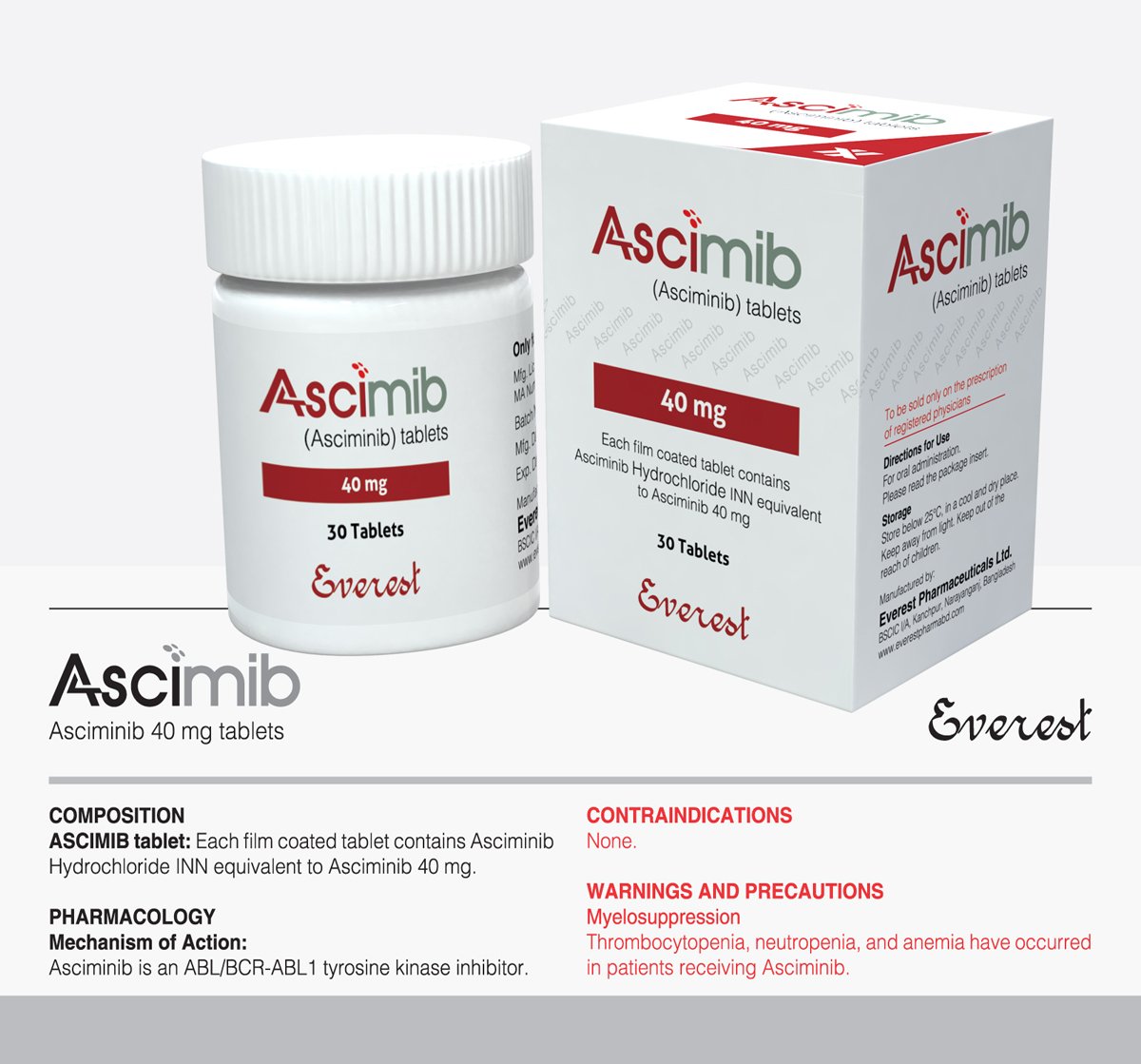


Reviews
There are no reviews yet.